Trial dose of the vaccine injected on 45 healthy individuals from Seattle
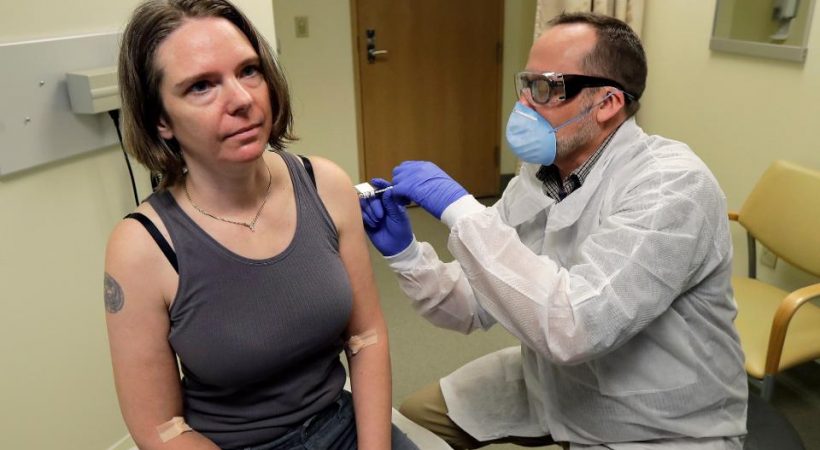
A person from Seattle has enrolled to receive the first trial dose of the potential vaccine for the SARS-CoV2 coronavirus. The vaccine, mRNA-1273, was developed by biotechnology company Moderna along with researchers from the National Institutes of Health (NIH). The trial is being conducted at Kaiser Permanente Washington Health Research Institute in Seattle.
As the initial trial, the first phase of the study will test three different doses of the mRNA-1273 vaccine on 45 healthy adults. All of them will receive two shots of the vaccine, 28 days apart and will be monitored to understand the safety and immunogenicity levels of the vaccine. Immunogenicity means testing the immunity level of the vaccine to a protein on the SARS-CoV2 coronavirus surface.
The first person on whom the trial was conducted is Jennifer Haller, a 45-year-old from Seattle. In an interview with TIME, she said, “I hope that we get to a working vaccine quickly and that we can save lives and people can go back to life as soon as possible.”
“This study is the first step in the clinical development of an mRNA vaccine against SARS-CoV-2, and we expect it to provide important information about safety and immunogenicity,” said Tal Zaks, M.D., Ph.D., Chief Medical Officer at Moderna in a press release. He added that Moderna, FDA and other organizations are working together to get ready for a phase 2 trial that would involve more number of patients.
The trial started 65 days after the Chinese authorities sequenced the SARS-CoVs coronavirus. Two days later, the researchers at the Vaccine Research Center at the NIH finalized the vaccine design and started working on its manufacture. Finally, they came out with the first batch on February 7th. After the analytical testing the vaccines were shipped to the NIH on February 24th.
“Finding a safe and effective vaccine to prevent infection with SARS-CoV-2 is an urgent public health priority,” said Anthony S. Fauci, M.D., head of the National Institute of Allergy and Infectious Diseases, at the NIH. “This Phase 1 study, launched in record speed, is an important first step toward achieving that goal,” he added.
The vaccine developed prevents the infection of COVID-19 and does not contain the virus as is the case with many other vaccines. What it contains is a small piece of genetic code, the mRNA that was extracted by scientists from the virus and later expanded in the laboratory.
Here, the mRNA encodes the viral spike protein which is necessary for the coronavirus to access the human cells. However, the researchers hope that the vaccine will stimulate the immune system and thus prevent the attack from the COVID-19.
Previously, the mRNA-1723 vaccine was not tested in mice before the human trials. This is something rare and has become controversial too. Experts insist that the severity and the urgent need of the situation demands that this is justified although others claim that this could break various ethical and safety standards and place trail participants at a greater risk than usual.
Although the prospective vaccine was designed and produced very quickly its evaluation is known to be more time consuming. All of the participants on whom the first trial was conducted will be observed for 12 months after the second vaccination to collect the data researchers initially need to figure out whether it is safe and effective.















